By Daniel Johnson, DO and Jordan Schooler, MD, PhD
Clinical Case
You arrive on the scene of a cardiac arrest. You find a middle-aged male with CPR in progress by bystanders. You find that he is in ventricular fibrillation. After quality CPR and defibrillation, you obtain ROSC. The patient remains comatose. You obtain a definitive airway and provide post-cardiac arrest care. Should this include the initiation of therapeutic hypothermia?
Background
The notion of therapeutic hypothermia dates back centuries. The concept of decreasing core temperature to prolong the time in which the body can endure hypoxia is appealing to those treating conditions involving tissue ischemia. Surgeons in the 1950’s noted that induction of hypothermia can decrease neural injury in canine models of cardiac surgery (1). Currently, cranial cooling is a mainstay of treatment in neonates suffering from hypoxic ischemic encephalopathy (2). The description of the pathophysiology is largely two-fold. Primarily, with decreasing temperature comes decreased metabolic demands. Additionally, enzymatic reactions which drive the process of ischemia-reperfusion injury are slowed at lower temperatures (1). While these concepts seem promising in a lab, the lack of uniform translation to clinical medicine leaves many questions unanswered.
If therapeutic hypothermia is effective in animal models, and in neonates, should it be included in the treatment of cardiac arrest?
After all, the arrest of spontaneous circulation is the near definition of an ischemic insult. With that being said, is it reasonable to theorize that cooling the victims of cardiac arrest could serve to decrease neurologic injury and ischemia-reperfusion injury in the event of return of spontaneous circulation (ROSC)?
The first two randomized controlled trials (RCTs) to address this very question were published in 2002. The Hypothermia after Cardiac Arrest Study Group published a trial of 275 participants suffering from out-of-hospital cardiac arrest (OHCA) due to a shockable rhythm (3). The intervention group received targeted temperature management to 33°C compared to the control group of normothermia (37°C). They found both improved survival and survival with favorable neurologic outcome (primary outcome) in the hypothermia group. Bernard et al. also published an RCT of 77 patients, also comparing 33°C vs. 37°C degrees, again with the primary outcome of neurologically favorable outcome, though in this case only including patients with OHCA due to ventricular fibrillation (4). They too found improvement in the primary outcome in the hypothermia group.
After these two “landmark” RCTs, there was widespread adoption of targeted temperature management (TTM) for treatment of comatose patients after cardiac arrest.
Do we really need to cool patients to 33°C or is 36°C effective?
As TTM became more widely utilized, this raised the question of ideal target temperatures, specifically whether actual hypothermia was necessary or merely the avoidance of fever. This was tackled by Nielsen et al. in the TTM Trial published in the New England Journal of Medicine in 2013 (5). They included 939 patients who suffered OHCA with a “presumed cardiac cause” and randomized them to 33°C or 36°C. With a primary outcome of all-cause mortality, they found no difference between the groups. Secondary outcomes of neurologic function also failed to reach statistical significance. Given that hypothermia in and of itself has associated risks, including increased incidence of cardiac arrhythmia, this trial suggests that a more modest target temperature of 36°C would be reasonable.
If TTM in the hospital is good, is initiation of hypothermia in the field better?
Two RCTs examined the utility of the initiation of hypothermia in the field. Bernard et al. enrolled 234 patients in a trial that compared cooled IV fluids during transportation by EMS versus standard care (6). When examining the primary outcome of survival to hospital discharge, there was no difference between the groups. This study was followed by Kim et al. who compared cooling with 2L of 4°C saline compared to usual care by EMS (7). While they reported no difference in the primary outcomes of hospital discharge and neurologic function, patients in the intervention group showed increased rates of re-arrest, pulmonary edema on first chest x-ray, and need for diuresis. These two RCTs related to prehospital initiation of hypothermia show no difference, if not harm, to patients.
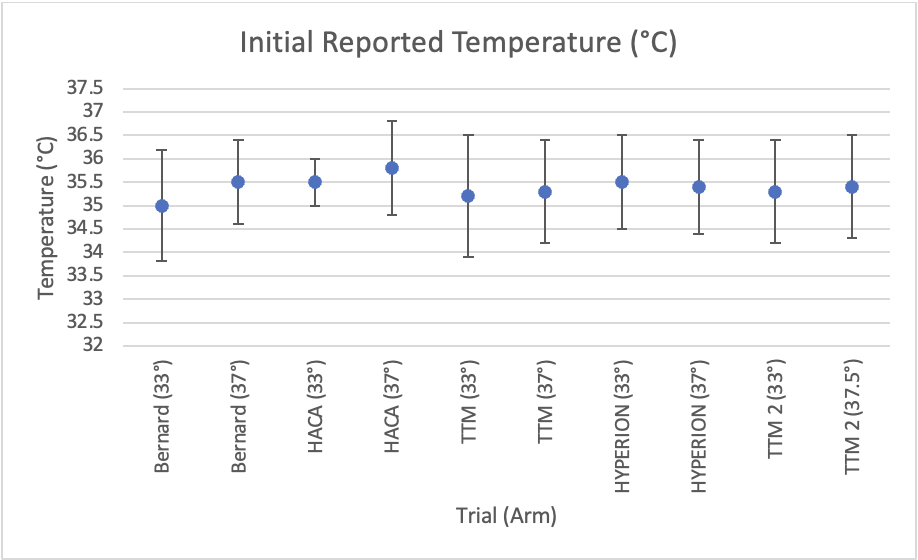
Figure 1: Temperature upon hospital presentation
Additionally, when examining the initial recorded temperature in each of the RCTs not involving prehospital cooling (Figure 1), patients did not arrive to the hospital hyperthermic. If the true benefit of TTM is not to target a sub-physiologic temperature, but simply to avoid hyperthermia, no intervention would be required in the prehospital setting to deliver patients to the ED in this state. Given the lack of benefit, potential harm, and lack of pre-hospital hyperthermia noted in these studies, it seems reasonable to keep TTM a therapy performed in the hospital.
Are we really sure that TTM works?
The two early trials of TTM noted above had methodologic flaws including small sample sizes and lack of blinding. Many in the scientific community questioned whether their results would be reproducible. The HYPERION Trial in 2019 included 581 patients with non-shockable rhythms and notably included in-hospital cardiac arrest patients (8). They compared TTM at 33°C versus normothermia targeted to 37.5°C and found that the hypothermia group had improved survival with favorable neurologic outcome (though no improvement in overall survival). In 2021, Dankiewicz et al. published TTM2, the largest RCT to date examining TTM in patient with OHCA from a presumed cardiovascular cause (9). They enrolled an astonishing 1861 patients (the initial two trials in 2002 enrolled a total of 352 patients) and compared TTM to 33°C versus targeted normothermia at 37.5°C. While the specificity of “targeted normothermia” in the control group may seem trivial, it is important to note that the development of fever during the post-resuscitation phase has been proposed as the true evil mitigated by TTM. In fact, a significant proportion of patients in the “normothermia” control group in the initial trials were actually febrile. In this study, no difference was found between the study groups in the primary and secondary outcomes of survival at 6 months and positive neurologic outcomes.
To summarize, we now have two small RCTs that ignited the idea that TTM is beneficial in the treatment of survivors of OHCA. Since then, we have established that rapid boluses of cool IV fluids in the prehospital setting are not beneficial and may be harmful. Even more significantly, the largest, and strongest trial to date did not replicate these initial findings. It is very likely that the initial positive findings were due to bias in the studies, and that there is no clinical benefit to hypothermia after cardiac arrest.
Despite the literature, what do the guidelines say?
Professional societies have created guidelines surrounding the use of TTM. In the latest American Heart Association guidelines, which were created prior to the publication of TTM2, they give Level 1, B-R recommendations to utilize TTM in adult patients who are comatose after return of spontaneous circulation in those suffering from OHCA with any initial rhythm (shockable or non-shockable). They also, notably, do not recommend the routine use of cold IV fluids in the prehospital setting (10).
For example, in the Commonwealth of Pennsylvania, Statewide ALS Protocols do address the topic of prehospital initiation of cooling. They acknowledge that prehospital cooling may be ordered by a medical command physician but specifically recommend this be obtained with external cooling mechanisms. They echo the concerns of Kim et al. and cite that rapid IV administration of cold fluids can results in pulmonary edema and recurrent cardiac arrest.
So, if tomorrow we find ourselves in the midst of a comatose, resuscitated patient after a ventricular fibrillation cardiac arrest – what should we do?
There are a few things we know that we should do after ROSC is obtained. Avoid hypotension, over-ventilation, and hypoxia. Titrate oxygenation between 95 and 99% to avoid overzealous oxygenation. Obtain a 12-lead ECG as soon as possible and consider resources for intervention if ST elevation MI is identified. These are the mainstays of good, post-ROSC care.
As far as cooling, while external cooling can be done if agreed upon with a medical command physician, given the lack of observed benefit, this is only likely to distract from more important aspects of patient care. There is evidence of harm associated with rapid IV administration of cooled fluids and this should be avoided. If possible, transport these patients to centers that can provide robust, multidisciplinary care for post-cardiac arrest patients. As it stands now, this will likely include TTM, but that may shift to simple avoidance of fever as the TTM2 results are absorbed into practice.
Authors: Daniel Johnson, DO | Assistant Professor, Department of Emergency Medicine, Life Lion EMS & Critical Care Transport, Penn State Health Milton S. Hershey Medical Center. Jordan Schooler, MD, PhD | Assistant Professor, Department of Emergency Medicine, Heart and Vascular Institute Critical Care Unit, Penn State Health Milton S. Hershey Medical Center
Editing by EMS MEd Editor James Li, MD (@JamesLi_17)