Summary and analysis by Maia Dorsett, MD, PhD @maiadorsett

Framing the Problem
Nausea and vomiting in pregnancy is not rare, occurring in about 50% of all pregnant women. [1] Symptom onset typically occurs within four weeks of the last menstrual period and peaks at nine weeks of gestation, with the vast majority of cases resolving by 20 weeks. The most severe form, hyperemesis gravidarum, is characterized by persistent vomiting, weight loss of more than 5%, ketonuria, electrolyte abnormalities and dehydration. [1] This occurs in a minority of patients (0.3 to 1.0%), although patients with a less severe form may also access the emergency department by utilization of ambulance services. [1] The frequency of EMS utilization for patients with hyperemesis gravidarum is unclear.
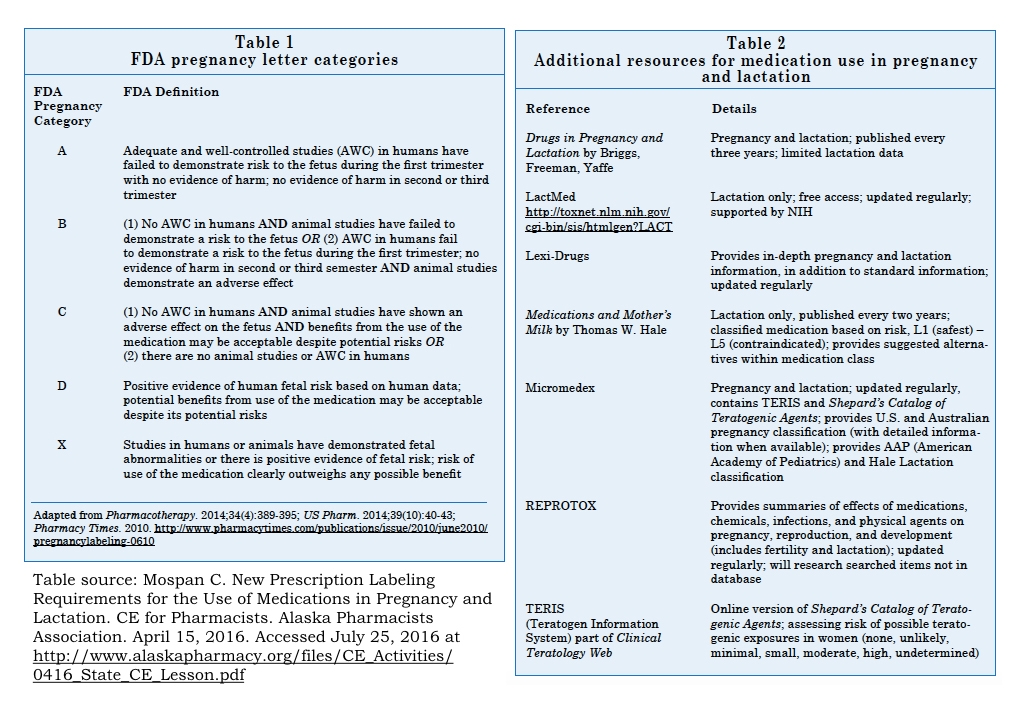
With regard to the most effective treatment of hyperemesis, the proverbial wind has blown in several directions. Lessons learned from the tragedy of thalidomide, used in the late 1950’s as a treatment for nausea in pregnant women before being banned in 1961 due to severe teratogenic effects, have raised the bar of safety considerations for the administration of medications to pregnant women. [2] Partially in response to the thalidomide tragedy, in 1979 the FDA established five letter risk categories – A, B, C, D or X – to help clinicians evaluate the risk versus benefit of medication use in pregnancy (see Figure, [3]). However, the pregnancy letter categories were often falsely interpreted to be an interpretation of teratogenic risk, leading to misunderstanding among clinicians and patients. Recently, the FDA has recommended eliminating the letter categories in favor of the Pregnancy and Lactation Labeling Final Rule (PLLR) that went into effect on June 30, 2015 . The PLLR requires a description of the “risk summary” and associated data regarding safety or harm in pregnancy and lactation.
The anti-emetic ondansetron is approved by the FDA for the treatment of nausea and vomiting associated with chemotherapy or surgery. However, its effectiveness at treating nausea and vomiting with little sedating side effects, as well as its classification as pregnancy category “B”, has now made it the most commonly used prescription oral antiemetic in pregnancy (at least in 2008). [4,5] More than half of women treated in emergency departments will receive intravenous ondansetron. [4,6] Retrospective cohort studies published in 2013 and 2014 came to conflicting conclusions regarding the safety of ondansetron in pregnancy, with one article finding a slight increase in the risk of cardiovascular defects. [see below for more in-depth review; 7,8] In September 2015, the American Society of Obstetricians and Gynecologists published a Practice Bulletin on Nausea and Vomiting in pregnancy. This practice bulletin recommended doxylamine and pyridoxine as first line treatment, and wrote that:
“There are insufficient data on fetal safety with ondansetron use and further studies are warranted…. Thus, although some studies have shown an increased risk of birth defects with early ondansetron use, other studies have not and the absolute risk to any fetus is low. As with all medications, the potential risks and benefits should be weighed in each case.” [5]
From the perspective of the EMS provider, should we giving ondansetron to pregnant women in their first trimester with nausea and vomiting? Our discussion forum reviewed one such case.
Case and Discussion
The Case:
EMS is called to the house of a 24 yo female who is 8 weeks pregnant. She has had countless episodes of nausea and vomiting over the last 4 days. She has been unable to take anything by mouth over this period and has not urinated in the last day. Heart rate is 115, blood pressure 101/60. The crew starts an IV and begins administration of IV fluids. The patient asks if they can give her anything for the nausea on the way to the hospital. The current protocol for nausea and vomiting recommends administration of 4 mg of IV ondansetron. Should the paramedics give it?
Multiple perspectives/comments regarding the case were shared (thanks to all who contributed!). These comments can be separated into a number of points or themes:
Theme #1: Outpatient treatment of nausea and vomiting is a clinically distinct situation from the acute management of intractable vomiting.
“I\’d just be careful with that guideline. I generally do use B6 & pyridoxine but that is after they stop vomiting. I think people with some nausea & vomiting who can manage their symptoms at home are different than those that are coming to the ED as they can\’t stop vomiting and need a SL or IV medication.” – E. Schwarz
“I agree with Evan that pyridoxine (vitamin B6) is helpful in reducing the tendency toward nausea & vomiting in pregnancy, but it is not an immediately effective treatment for HG even when given IV (which I sometimes do since the woman may have continued difficulty with oral intake and absorption).” – M. Mullins
This is a really important point. We often inappropriately extrapolate general recommendations from one clinical situation and apply them to another. The ACOG guidelines refer to treatment of nausea and vomiting in pregnancy, the majority of which is treated at home. The patient population using EMS for refractory vomiting and/or have hyperemesis gravidarum is different from those who would tolerate oral doxylamine and pyridoxine. Even when it comes to oral treatment, one small randomized trial involving 36 women compared 5 days of treatment with ondansetron versus doxylamine/pyridoxine. [10] This study found that ondansetron was superior in reducing nausea/vomiting in pregnancy. The ACOG guidelines write that “the potential risks and benefits should be weighed in each case”, leaving room to use alternatives to doxylamine and pyridoxine in a patient who is not tolerating oral intake or with clinical signs of dehydration.
Theme #2: The data regarding ondansetron and birth defects is not particularly convincing and most EMS protocols do not distinguish between pregnant and non-pregnant patients when it comes to the treatment of nausea/vomiting.
“In addition, many are still not convinced that ondansetron is truly problematic in pregnancy.” – E. Schwarz
“no limitations to choice of anti-emetic at my agency.” – S. Pearson
“I remain unconvinced the Ondansetron is a cause of birth defects rather than being associated with problem pregnancies with more nausea. Although it may or may not be effective in HG, it is Category B and remains the first line anti-emetic for paramedics in the field.” – M. Mullins
“Our (statewide) protocols use ondansetron as first-line treatment for nausea/vomiting, and do not exclude or distinguish between pregnant and non-pregnant patients. The most recent data on the subject (Ondansetron in pregnancy and risk of adverse fetal outcomes in the United States. Reprod Toxicol. 2016 Jul;62:87-91.) is reassuring and throws into question the previously reported association with cardiac defects.” – M. Holtz
The overall prevalence of cardiac or major congenital anomalies is low, and therefore one should have larger sample sizes to detect an increase in teratogenic risk. To date, there are only three peer-reviewed and published studies with greater than 1000 pregnancies with ondansetron exposure that have evaluated for an association between ondansetron and birth defects. Given the inability to perform a randomized-control trial to evaluate for risk of birth defects due to ethical concerns, all three of these studies are retrospective cohort studies.
1. Pasternak et. al. (2013) [7]: This was a cohort study of 441,511 pregnancies in Denmark utilizing data from the National Patient Register and medical Birth Registry from 2004-2011. The authors studied women with ondansetron exposure prior to the 12th week of pregnancy (1233 pregnancies) and matched these to unexposed women (4,932 pregnancies). After adjusting for possible confounding variables (including hospitalization for hyperemesis and maternal comorbidities including diabetes), the authors did not find any significant association between ondansetron-exposure and the incidence of major birth defects (OR 1.12, 9%% 0.69 – 1.82) or cardiovascular defect (OR 1.04, 95% CI 0.52 – 1.95).
2. Danielsson et. al. (2014) [8]: This retrospective cohort study in Sweden identified women through midwife interviews at the first antenatal visit and through the Swedish Prescription Drug registry. The study included approximately 1.5 million births, with 1349 ondansetron-exposed infants. They did not find a significant difference in risk of “major malformation” (OR 1.11, 95% CI 0.81-1.53). However, they did find a slightly increased risk of cardiovascular defects in the ondansetron-exposed group (OR 1. 62, 95% CI 1.04-2.14).
3. Fejzo et. al. (2016) [11]: This was a retrospective cohort study of women in the United States recruited through the Hyperemesis Education and Research Foundation between 2007 and 2014. Normal controls were recruited by study participants. The study recruited women in three groups: (1) Hyperemesis with ondansetron (1070 pregnancies), (2) Hyperemesis without ondansetron (771 pregnancies), and (3) No hyperemesis without ondansetron (1555 pregnancies). Participants filled out online surveys on fetal outcome following their due date. They found that the rate of birth defects was equally reported among the HG groups (3.47% in HG/ondansetron group vs. 3.40% in HG/no ondansetron group, p = 1.0), and increased in comparison to the control group (1.87%), suggesting that birth defects may be associated with problem pregnancies with more nausea. They also found that women with a history of HG who took ondansetron were significantly less likely to report miscarriage or termination of their pregnancy due to hyperemesis gravidarum. This study was limited by potential recall bias, as these were all self-reported outcomes.
The combination of dicyclomine and pyridoxine was transiently taken off the market in 1983 because of allegations of teratogenicity only to be reinstated later as first line therapy and the first FDA-approved treatment for hyperemesis gravidarum [1]. We will see what the future holds for ondansetron, but the above evidence is far from convincing of direct teratogenic effects of ondansetron-exposure rather than confounding effects.
Theme#3: What about alternatives?
“Actually, if you look at a drug\’s mechanism of action, metoclopramide makes a lot more sense than ondansetron. Metoclopramide lowers GI esophageal sphincter tone and speeds gastric emptying, precisely the opposite of what progesterone does to the pregnant woman\’s GI system. It puzzles me that we target antibiotics mechanistically to treat microbes, yet we don\’t target anti-emetics to treat nausea based on the presumed cause. I\’m just sayin…. I have treated several ondansetron-refractory ladies with metoclopramide with excellent results (And by the way, if you are worried that Metoclopramide may not be as “safe” as ondansetron, perish the thought. Metoclopramide, just like ondansetron, is “Class B”)” – G. Gaddis
Within the scope of retrospective data, Metoclopramide appears to be safe in pregnancy. A retrospective cohort study involving 113, 612 pregnancies with 3458 exposures to metoclopramide in the first trimester of pregnancy found no increased risk of adverse outcomes. [12] In a randomized-control trial of ondansetron and metoclopramide for hyperemesis gravidarum, the two drugs performed comparably in nausea-reduction, but there was more reported side-effects of drowsiness and dry mouth in the metoclopramide-treated group. [13]
Take Home Points

The evidence that ondansetron causes harm in pregnancy is far from conclusive. As EMS takes care of women with more severe symptoms, intravenous ondansetron can be considered as a therapy in pregnant women who present with intractable nausea and vomiting.
References:
1. Niebyl, J. R. (2010). Nausea and vomiting in pregnancy. New England Journal of Medicine, 363(16), 1544-1550.
2. Kim, J. H., & Scialli, A. R. (2011). Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicological Sciences, 122(1), 1-6.
3. Mospan C. New Prescription Labeling Requirements for the Use of Medications in Pregnancy and Lactation. CE for Pharmacists. Alaska Pharmacists Association. April 15, 2016. Accessed July 25, 2016 at http://www.alaskapharmacy.org/files/CE_Activities/0416_State_CE_Lesson.pdf
4. Siminerio, L. L., Bodnar, L. M., Venkataramanan, R., & Caritis, S. N. (2016). Ondansetron use in pregnancy. Obstetrics & Gynecology, 127(5), 873-877.
5. Mitchell, A. A., Gilboa, S. M., Werler, M. M., Kelley, K. E., Louik, C., Hernández-Díaz, S., & Study, N. B. D. P. (2011). Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. American journal of obstetrics and gynecology, 205(1), 51-e1.
6. Mayhall, E. A., Gray, R., Lopes, V., & Matteson, K. A. (2015). Comparison of antiemetics for nausea and vomiting of pregnancy in an emergency department setting. The American journal of emergency medicine, 33(7), 882-886.
7. Pasternak, B., Svanström, H., & Hviid, A. (2013). Ondansetron in pregnancy and risk of adverse fetal outcomes. New England Journal of Medicine, 368(9), 814-823.
8. Danielsson, B., Wikner, B. N., & Källén, B. (2014). Use of ondansetron during pregnancy and congenital malformations in the infant. Reproductive Toxicology, 50, 134-137.
9. Goodwin, T. M., & Ramin, S. M. (2015). Practice Bulletin Summary No. 153: Nausea and Vomiting of Pregnancy. OBSTETRICS AND GYNECOLOGY, 126(3), 687-688.
10. Oliveira, L. G., Capp, S. M., You, W. B., Riffenburgh, R. H., & Carstairs, S. D. (2014). Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstetrics & Gynecology, 124(4), 735-742.
11. Fejzo, M. S., MacGibbon, K. W., & Mullin, P. M. (2016). Ondansetron in pregnancy and risk of adverse fetal outcomes in the United States. Reproductive Toxicology, 62, 87-91.
12. Matok, I., Gorodischer, R., Koren, G., Sheiner, E., Wiznitzer, A., & Levy, A. (2009). The safety of metoclopramide use in the first trimester of pregnancy. New England Journal of Medicine, 360(24), 2528-2535.
13. Abas, M. N., Tan, P. C., Azmi, N., & Omar, S. Z. (2014). Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstetrics & Gynecology, 123(6), 1272-1279.